Edible oils: an introduction – The multi-faceted personality of our cooking oils
(Refer Post 2: HEALTH, HAPPINESS, LIFE AND FOOD (PART I) : WHAT ARE THEY? (letfoodliftlife.com), Post 4: INDUSTRIAL PROCESSED FOODS: PART I, THEIR EVOLUTION AND PRESENT STATUS (letfoodliftlife.com) and, Post 16: OXYGEN, FOOD AND LIFE : PART II (THE DARK SIDE OF OXYGEN) (letfoodliftlife.com) to understand how they are vulnerable to harmful attack by air during storage, cooking and industrial processing.)
 Introduction: ‘Oil’ is attached to many things in our life: petroleum oil, paraffin oil, lubricating oil, essential oil, spice oil, synthetic oil, edible oil etc. They are all chemically different but share the broad characteristics of being light, viscous and relatively non-volatile, except some essential oils. Here we are talking about edible oils (groundnut oil, refined soybean oil, olive oil, palm oil) that we use in daily cooking in many ways and processors use in churning out a variety of processed foods. Sometimes we use them as direct accompaniments to dishes that need some flavor support and lubrication in the mouth.
Introduction: ‘Oil’ is attached to many things in our life: petroleum oil, paraffin oil, lubricating oil, essential oil, spice oil, synthetic oil, edible oil etc. They are all chemically different but share the broad characteristics of being light, viscous and relatively non-volatile, except some essential oils. Here we are talking about edible oils (groundnut oil, refined soybean oil, olive oil, palm oil) that we use in daily cooking in many ways and processors use in churning out a variety of processed foods. Sometimes we use them as direct accompaniments to dishes that need some flavor support and lubrication in the mouth.
 Their natural sources: They can be both plants and animals; in India animal source oils are not used for cooking or direct consumption. These are the oils that ooze out during the cooking of some meats and are ‘rendered’ out of their animal sources industrially in some countries. They tend to be ‘fats’ rather than oils (see the ‘distinction’ below) and, unlike vegetable source oils, contain animal-source constituents like vitamin D and cholesterol.
Their natural sources: They can be both plants and animals; in India animal source oils are not used for cooking or direct consumption. These are the oils that ooze out during the cooking of some meats and are ‘rendered’ out of their animal sources industrially in some countries. They tend to be ‘fats’ rather than oils (see the ‘distinction’ below) and, unlike vegetable source oils, contain animal-source constituents like vitamin D and cholesterol.
Plant-source oils can be of tree origin (e.g. palm, coconut and olive oils) or seed origin (e.g. peanut/groundnut oil, sesame/til oil, cottonseed oil, sunflower oil, soybean oil…etc.). Rice bran oil is a part of the outer coat or bran of rice grain and wheat germ oil and corn oil are found in the ‘germ’ (a small reproductive part of the grain) of wheat and corn. Despite this variety in their origin in nature, they have similar chemical composition and physical properties while retaining their distinct identities in finer nuances. It is an intricate world being simplified here.
 Oils and fats, a superfluous distinction: Oils and fats have the same broad chemical compositions with differences in minor details which change their physical form. Generally, fats are solid (actually, solid-looking) at normal temperatures, e.g. lard, tallow, kokum fat, mango kernel fat, sal fat, ghee… etc. while oils are liquid e.g. sesame oil, cottonseed oil, soybean oil, groundnut oil..etc. But this is a superfluous distinction: coconut oil can be quite solid during winter and all the fats listed above are quite liquid during Central India summers. Palm oil is a special oil that has a near-equal mixture of liquid and solid parts and its liquid part is not called palm oil (but palmolein) and the solid part, not palm fat (but palm stearine). An oil can become solid on chemically modifying it by hydrogenation but it is still called hydrogenated groundnut oil or soybean oil rather than groundnut fat or soybean fat. We will consistently call them all, ‘oils’. For physiological reasons, bodily lipids are referred to as fats, perhaps for convenience.
Oils and fats, a superfluous distinction: Oils and fats have the same broad chemical compositions with differences in minor details which change their physical form. Generally, fats are solid (actually, solid-looking) at normal temperatures, e.g. lard, tallow, kokum fat, mango kernel fat, sal fat, ghee… etc. while oils are liquid e.g. sesame oil, cottonseed oil, soybean oil, groundnut oil..etc. But this is a superfluous distinction: coconut oil can be quite solid during winter and all the fats listed above are quite liquid during Central India summers. Palm oil is a special oil that has a near-equal mixture of liquid and solid parts and its liquid part is not called palm oil (but palmolein) and the solid part, not palm fat (but palm stearine). An oil can become solid on chemically modifying it by hydrogenation but it is still called hydrogenated groundnut oil or soybean oil rather than groundnut fat or soybean fat. We will consistently call them all, ‘oils’. For physiological reasons, bodily lipids are referred to as fats, perhaps for convenience.
Chemical nature of oils
Molecular constituents: Starch is made up of repeatedly chemically joined units of glucose and proteins, of repeatedly chemically joined units of various amino acids. On breaking down by reaction with water (hydrolysis), as in digestion in our system, they respectively produce glucose and amino acids. In nature, starch derives its variety from the number of glucose units per molecule and whether they are joined strictly in a line or whether there are branches or whether they are cross-linked across chains. Similarly, proteins are varied in terms of number of constituent amino acids and their variety per molecular chain. They are more complex than starches and have varying physical and chemical properties but it remains a basic truth that pure proteins hydrolyse into amino acids.
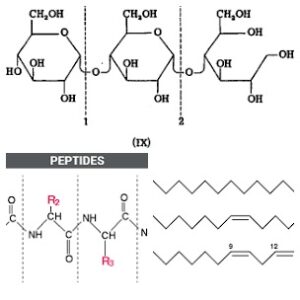
Glycerol (or glycerine in trade) is a straight molecule which has three hydroxyl (-OH) groups, each of which can chemically combine with same or different fatty acids. Such ‘tri-glycerides’ form the bulk of any oil. The differences in their chemical and physical properties stem from the fatty acids variety and their ‘arrangement’ on the glycerol molecule.
(An interesting comparison of the make-up of the three macronutrients of food: Starch, the most abundant nutritive carbohydrate in our food, is made up of just glucose molecules connected together. Cellulose, by far the most abundant carbohydrate – even organic compound – in nature, is also similarly made up of just glucose but in such a way that it remains insoluble in water and hence constitutes the bulk of insoluble fiber in our diet. Starch and cellulose account for the lion’s share of our carbohydrate intake; starch normally gets digested completely and vanishes from the GI tract by absorption into blood while cellulose reaches the colon unaffected. Visit post no. 2: HEALTH, HAPPINESS, LIFE AND FOOD (PART I) : WHAT ARE THEY? (letfoodliftlife.com).
Proteins are made up of only amino acids but of many varieties and hence their hydrolysis (or digestion) produces a mix of many amino acids.
Oils are made up of glycerol – a uniquely defined molecule like glucose – and a variety of fatty acids – like amino acids of proteins. Such a mix of triglycerides defines the oil and the variety in such mixes differentiates one oil from another. Their hydrolysis (or digestion) produces glycerol and a mix of many fatty acids – thus two products: glycerol and a mix of many fatty acids. Compare with the products of starch and protein hydrolysis.
Starch is dispersible in hot water. Proteins are generally not easily dispersible in water, though dissolution of some large classes can be increased thru addition of some agents in water. Oils are not miscible with water at all and the apparent intimate mixing with water with the help of emulsifiers still leads to a mixture, not a solution.
Starch, cellulose, proteins and oils constitute almost our entire food intake, not counting water that inevitably comes with the first three.)
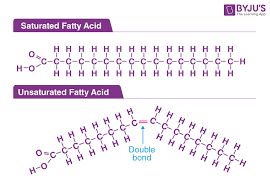
Fatty acids: They are fundamentally ‘saturated’ or ‘unsaturated’. The former generally have a straight chain structure as in hydrocarbons with all single carbon-carbon bonds which are stable and hence non-reactive. The latter have (usually, in most oils of practical interest) one, two or three double bonds between one, two or three different carbon pairs. Those with a double bond between only one pair of carbon atoms are called mono-unsaturated fatty acids (or MUFA) and the other two are called PUFA – polyunsaturated fatty acids with U standing for unsaturation or C-C double bonds.
The ones with two double bonds are the ‘omega 6’ fatty acids and those with three double bonds are the ‘omega 3’ fatty acids with some inconsequential exceptions. These ‘omega’ fatty acids are ‘essential’ because our body cannot make them and they must be supplied thru diet. This gives oils like soybean oil and canola oil, with their content of alfa linolenic acid (the omega 3 essential fatty acid) a special place among vegetable oils. Since double bonds or ‘unsaturation’ are unstable, the fatty acids (and the oils carrying them) are reactive at those sites.
 Longer chain omega 3 and 6 fatty acids than the common omega 3 and 6 fatty acids are found in fish oils and flaxseed oil and have 5 or even 6 double bonds. Understandably, these oils are liquid, are highly reactive and hence very susceptible to oxidative damage. They have been credited with some health benefits but conventional omega 3 and 6 fatty acids from seed oils can convert to longer chain fatty acids within our body. (Caution: roast flaxseeds carefully at home; lowest of the smallest flame, thick-bottomed pan, severely restricted duration and stirring with fingers (!) works. We will allot an entire post eventually to ‘domestic roasting’ of all kinds.)
Longer chain omega 3 and 6 fatty acids than the common omega 3 and 6 fatty acids are found in fish oils and flaxseed oil and have 5 or even 6 double bonds. Understandably, these oils are liquid, are highly reactive and hence very susceptible to oxidative damage. They have been credited with some health benefits but conventional omega 3 and 6 fatty acids from seed oils can convert to longer chain fatty acids within our body. (Caution: roast flaxseeds carefully at home; lowest of the smallest flame, thick-bottomed pan, severely restricted duration and stirring with fingers (!) works. We will allot an entire post eventually to ‘domestic roasting’ of all kinds.)
What unsaturated fatty acids do to oils: In nature, all plant source oils have PUFA and MUFA with double bonds that give the chain a ‘curved’ look at the double bond site with the result that tri-unsaturated fatty acid molecules look like a circular hook! Hydrogenation of oils either saturates the double bonds (i.e. single C-C bonds) or straightens double bonds which are still unsaturated. The latter are the infamous ‘trans fatty acids’ (TFA) which have been implicated in distortion of our blood lipid profiles and many resultant illnesses. This straightening of fatty acid chains allows them to be packed closer and gives them a solid appearance. Because of the attendant reduction in reactivity, they are stable, especially to oxidation (and rancidity) – saturated much more than trans.
Measuring unsaturation of fatty acids and hence of oils: Unsaturation gives fatty acids (and hence the oils of which they are parts) reactivity (or lack of stability) with oxygen. This makes them susceptible to rancidity – by far the commonest mode of ‘staling’ of oils and foods containing them. It also gives them liquid character which makes their daily handling easy – both at home and in the industry. The extent of unsaturation in oil is, therefore, an important quality parameter. It is measured in terms of a chemical property called ‘Iodine value’ or IV; the more the IV, the more the unsaturation, the more the liquidity and the more the susceptibility to oxidative rancidity. Not surprisingly, naturally solid oils like palm oil or hydrogenated oils with low IV are preferred when the oil (or the formulation that it is a part of) is to be heated as in frying and baking because it resists oxidative damage. This damage is further reduced by addition of synthetic anti-oxidants. Unrefined oils which have retained their natural anti-oxidants stay fresh longer.
The non-chemical nature of oils
Unrefined oils as mixtures: In nature, all oils are a mixture of tri-glycerides along with some di- and mono-glycerides and some ‘impurities’ – some of which are actually vitamins or vitamin analogues or precursors (A, D, E and K) and physiologically beneficial molecules like lecithin and anti-oxidants. During refining, most of these ‘impurities’ are removed giving us a good-looking, (rather than good!) ‘refined oil’. We need both, the good and good-looking types. (Refer to post no. 4: Industrial processed foods: their evolution and present status for more details.)
 Various ‘avatars’ of oil: In plants, oils exist in tiny droplets in special ‘cells’ from which they come out when steamed or cooked and pressed or extracted with solvents. Note that you are consuming edible oils at their best and the most natural when you munch on peanuts or groundnuts, sesame seeds or copra or coconut. But it is still oil so be careful. At this stage, they are ‘crude’ or ‘raw’ or ‘unrefined’ containing traces of water and the aforesaid ‘impurities’.
Various ‘avatars’ of oil: In plants, oils exist in tiny droplets in special ‘cells’ from which they come out when steamed or cooked and pressed or extracted with solvents. Note that you are consuming edible oils at their best and the most natural when you munch on peanuts or groundnuts, sesame seeds or copra or coconut. But it is still oil so be careful. At this stage, they are ‘crude’ or ‘raw’ or ‘unrefined’ containing traces of water and the aforesaid ‘impurities’.
The unrefined oils are loaded with constituents like plant pigments (most of which are anti-oxidants), gum-like useful substances, oil-soluble vitamins, some natural anti-oxidants, free fatty acids (ffa) detached from glycerides because of natural oil-hydrolysing enzymes (lipases), traces of sterols (called phyto-sterols because of plant origin unlike cholesterol originating in animals), stanols and a bunch of odour-contributing constituents etc. Of these, ffa need to be removed by refining; odour must be removed only in some cases (in India, flavourful unrefined oils are popular) but refining removes almost everything. This makes the oils lighter in colour and odourless but a bit ‘feeble’.
When refined, removal of ‘impurities’ boosts proportion of glycerides which remain unaffected. Refined soybean oil contains 99% (or even more) tri-glycerides. Refined palm oil and rice-bran oil contain significant levels of mono- and diglycerides which have special properties including lower calories per gram. In Japan, oils with significant proportions of di-glycerides are popular as a ‘diet oil’.
All our discussions will be limited to unrefined and refined oils which covers most of our edible oil usage. A separate post will be dedicated to hydrogenated oils which have substantial functionalities even if loaded with TFA.
Refined vs. unrefined oils: Refined oils are bland and hence help prepare dishes and industrial products with ‘predictable’ flavor profiles. Since all refined oils look and feel pretty much the same, almost any refined oil can be used for any application, though our physiology and the need for protection from oxidation would impose certain restrictions. Lower in natural anti-oxidants, high IV refined oils (like soybean oil) are vulnerable to oxidation and attendant off-flavour development. This is why not all refined oils can be used freely in frying and baking. Refined, naturally low IV oils like palm oil and hydrogenated oils (with artificially lowered IV) are more suitable for these processes but they would create a ‘greasy’ product if the oil oozes out to the surface during storage. You have seen the supposedly attractive sweets with a layer of solidified ghee at the top.
Unrefined soybean oil, cottonseed oil, rice bran oil, sunflower oil, mustard oil etc would be difficult to consume directly because of their distinct odours, dark colours and high content of ‘free fatty acids – ffa’. As discussed in Post 4, some naturally low ffa oils in unrefined form are popular in India for direct consumption, something that deserves encouragement. Hearteningly, soybean oil is becoming popular in its ‘soft-extracted’ and unrefined form.
Palm oil is so rich in carotenes (mainly beta carotene) and vit E and its analogues that a by-product of its refining is a prized commodity for recovery of these constituents. Interestingly, ‘refined red palm oil’ is a special category of refined palm oil which retains its original red colour and hence those nutrients. It deserves to find more food uses.
Thus oils are hydrophobic (immiscible with water), about 10% lighter than water, more viscous and a source of valuable micro-nutrients. Their physiological functionalities are potentially life-saving and their food functionalities are delightful as we are about to find out. Our fear of them stems from misinformation (something to be allayed soon) but they can undoubtedly cause serious chronic ill-health if misused (as we will realize soon). How to leverage them for health and happiness is de-mystifying and empowering, also happening soon!
Next Post:
Edible oils have many roles outside our body
The non-physiological functionalities of edible oils
Visit Disclaimer
16 thoughts on “Edible oils: an introduction – The multi-faceted personality of our cooking oils”
The articles you write help me a lot and I like the topic
Please tell me more about your excellent articles
We have written three posts on edible oils. Feel free to check out the remaining two posts on edible oils.
Your articles are extremely helpful to me. Please provide more information!
Thank you for your post. I really enjoyed reading it, especially because it addressed my issue. It helped me a lot and I hope it will also help others.
You’ve been great to me. Thank you!
May I have information on the topic of your article?
I’m so in love with this. You did a great job!!
Please provide me with more details on the topic
Gladly. Kindly specify your queries here. Also, feel free to browse our blog for more details provided in related posts.
I enjoyed reading your piece and it provided me with a lot of value.
Thank you for your post. I really enjoyed reading it, especially because it addressed my issue. It helped me a lot and I hope it will also help others.
You helped me a lot by posting this article and I love what I’m learning.
You need to participate in a contest for among the best blogs on the web. I will advocate this site!
You have brought up a very great points, appreciate it for the post.