Oxygen, food and life : Part I (How oxygen mediates in life and food processing)
(Note: Visit post no. 2: Health, happiness, life and food, Part I, What are they? to understand what is meant by ‘life’ in this post and the next post to understand how life-giving oxygen can be life-threatening.)
Preamble: There are striking similarities between water and oxygen: (i) Both are chemically reactive and are found everywhere. (ii) Both make life itself possible in different ways. (iii) The properties and effects of both need to be controlled and modulated, failing which they can turn nasty. (iv)Though oxygen is strictly an element and water, a compound, both have simple molecular structures.

Oxygen in nature: Oxygen is a colourless, odourless gas, heavier than water vapour and much heavier than hydrogen. In free state, it is an intimate part of air around us along with nitrogen and traces of carbon dioxide, water vapour and a few other gases, not counting polluting particles. For our practical purposes, we can simplify air as a 1:4 mixture of oxygen and nitrogen, both in terms of number of molecules and volume. It is present in earth’s crust both as free oxygen and as chemically combined oxygen – by far most abundantly as silica. In vegetation, it exists in combined form as the most abundant natural organic polymer – cellulose. Many natural minerals of metals exist as their oxides i.e. combined with oxygen or as sulphides i.e. combined with sulfur – a ‘family member’ of oxygen.
 Solubility of oxygen in water is poor, about 8 mg per liter water at 250C, that too after saturation i.e. when the contact of air with water has been intimate and long. Hence, while oxygen is everywhere, it can’t find place within water. It is almost as if they have marked out their territories and won’t mess with each other! Not surprisingly, it is even less in the watery part of blood which already has a lot else dissolved in it. Most of the oxygen in blood is chemically bound to haemoglobin (found in red blood cells) because it has chemical affinity for oxygen. This oxygen is off-loaded at the cells in body tissues where it performs its designated role of oxidizing sugars and producing energy thru an extremely complex mechanism. This is the reason why physicians look for haemoglobin content of blood in a patient complaining of weakness; poor haemoglobin levels means poor oxygen-carrying capacity and poor availability of energy by combustion.
Solubility of oxygen in water is poor, about 8 mg per liter water at 250C, that too after saturation i.e. when the contact of air with water has been intimate and long. Hence, while oxygen is everywhere, it can’t find place within water. It is almost as if they have marked out their territories and won’t mess with each other! Not surprisingly, it is even less in the watery part of blood which already has a lot else dissolved in it. Most of the oxygen in blood is chemically bound to haemoglobin (found in red blood cells) because it has chemical affinity for oxygen. This oxygen is off-loaded at the cells in body tissues where it performs its designated role of oxidizing sugars and producing energy thru an extremely complex mechanism. This is the reason why physicians look for haemoglobin content of blood in a patient complaining of weakness; poor haemoglobin levels means poor oxygen-carrying capacity and poor availability of energy by combustion.
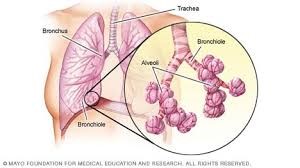
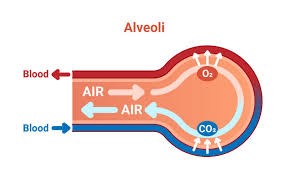
How oxygen enters and exits our body and makes life happen: When we breath in the air, oxygen (along with nitrogen) reaches our lungs thru the wind-pipe (trachea) which branches profusely into tiny tubes called bronchi (bronchus in singular) as it enters the lungs. These become extremely thin ‘bronchioles’ when branched maximally at the end of which are tiny, thin-walled sacs called ‘alveoli’. Surrounding these alveoli are equally thin-walled blood vessels into which oxygen diffuses, to ‘oxygenate’ blood. These thin vessels then ‘regroup’ or ‘reverse branch’ into progressively larger vessels carrying progressively larger flow of blood till a single blood vessel (formed by merging of a branch each from either lung) returns the now ‘oxygenated blood’ to the heart.
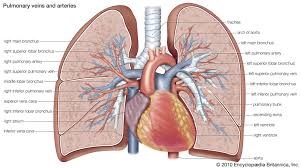 Heart pumps it out to all corners of the body, the first of which is the heart wall itself, thru the ‘coronary artery’! The organs controlled by the Central Nervous System like the heart, lungs, liver, kidneys, pancreas etc. (i.e. those that work on their own unlike hands and feet) also use oxygen delivered to them thru blood. It reaches the cells of our body along with co-traveller, glucose – remember the ‘serum glucose level’ of, say, 100 mg/dL? How the oxygen-sugar duo travels across the wall of the cells and ultimately reacts (or the sugar ‘burns’) within the cells is the stuff of divinity. This reaction of oxygen with sugar releases energy that enables us to do everything we do (including the use of brain) and maintains body temperature. Unfortunately, the escorting of the sugars into cells requires one more player called ‘insulin’, but that’s another story.
Heart pumps it out to all corners of the body, the first of which is the heart wall itself, thru the ‘coronary artery’! The organs controlled by the Central Nervous System like the heart, lungs, liver, kidneys, pancreas etc. (i.e. those that work on their own unlike hands and feet) also use oxygen delivered to them thru blood. It reaches the cells of our body along with co-traveller, glucose – remember the ‘serum glucose level’ of, say, 100 mg/dL? How the oxygen-sugar duo travels across the wall of the cells and ultimately reacts (or the sugar ‘burns’) within the cells is the stuff of divinity. This reaction of oxygen with sugar releases energy that enables us to do everything we do (including the use of brain) and maintains body temperature. Unfortunately, the escorting of the sugars into cells requires one more player called ‘insulin’, but that’s another story.
Since oxygen is consumed in burning glucose, blood is now ‘de-oxygenated’ and hurries back to the heart to be pumped to the lung where the entering single blood vessel (a branch to each lung) branches profusely (a lot like the bronchi) to become the thin-walled blood vessels hugging the alveoli to pick up breathed-in oxygen. And on and on it goes making life happen. Thru oxygen from air and water in the blood replenished by the water you drink. Nitrogen has no haemoglobin looking for it so it is left to fend for itself in the blood at a negligible and inconsequential level. It cannot enter blood thru pure dissolution (unlike oxygen which chemically latches on to haemoglobin i.e. is pulled in) because blood is generally ‘saturated’ with nitrogen – though at a miniscule level because of poor solubility.
The route of oxygen in our body can be represented as: lungs to circulating blood within the lungs to heart to body parts to back to heart to lungs…..When oxygen supply is limited for whatever reason, energy level drops. When working intensely in a poorly ventilated room, always go to the window and take deep breaths every now and then. Also, working out is best in an open ventilated area. Unused oxygen, along with carbon dioxide produced by sugar burning, traces the path exactly reverse to incoming air, till it leaves the body when we exhale. (Water is also co-produced during combustion but that is a part of the body!)
Note that the blood pathway is: deoxygenated blood from the body to heart to lungs (then oxygenated blood) to heart to the body, i.e. the lungs are the blood-oxygen meeting spot!

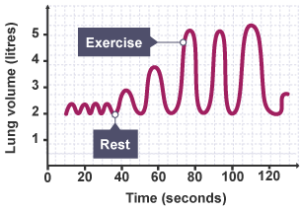
Working out, slimming and oxygen: The above segment shows why oxygen is called ‘praan-vayu’ in most Indian languages. In case of extreme or sustained muscular work, all our voluntary muscles use up the blood supply of glucose (when its level dips, it can come from glycogen – a water-insoluble glucose polymer – stored in the liver), glucose released by breakdown of muscles’ own stored glycogen, fat stored in their own adipose tissue, then the subcutaneous (below the skin e.g. around the tummy) adipose tissue and ultimately proteins themselves. This is the basis of slimming thru aerobic exercise, ideally combined with smart dieting. But oxygen remains common as the reactant that ‘burns’ these chemical energy sources one by one for energy release. Note that you need to thank oxygen even for slimming!
When you work out, the body responds by increasing breathing rate i.e. oxygen intake rate and heart rate i.e. blood circulation rate. This ensures more and more blood picking up more and more oxygen for more and more energy release. Such increase happening smoothly in proportion to requirement and returning to normal quickly at the end of the requirement, is a sign of good heart-lung health. Roboticists can make robots dance to their tunes; will they ever be able to breathe ‘life’ into them?!
You can see life taking shape here in the interactions between the body and the environment and, within the body, among various organs. Obviously, oxygen can do nothing without the agency of lungs transferring it to blood and blood is helpless unless mobilized by the untiring exertions of the heart.
Oxygen in food processing: The above-mentioned ‘reactivity’ of oxygen is usually a problem in food processing where it can cause undesirable reactions with food constituents, damaging the food and hence, the strategy is to keep oxygen (or air) out in most cases. But the same reactivity is sometimes exploited where the strategy is to ‘manage’ its access to the food being processed so that the reactions happen in the planned way.
Roasting of coffee beans: When hot coffee is raised to your lips and sipped, the experience is described evocatively as its ‘bouquet’. Most of it comes from the coffee powder and no less than 500 distinct chemicals have been identified as responsible for this! This ‘coffeeness of coffee’ is created when its beans are roasted in specially designed roasters under tightly controlled conditions of heating, stirring, presence of air (i.e. oxygen) and roasting time. Each variety has its own roasting specifications, without significant variations across varieties, of course.


Importantly, roasted coffee is ideally ground closest to the consumption time because the extensive surface area exposed by grinding makes it vulnerable to the effect of the atmosphere. This is the reasons why Americans buy their coffee beans roasted (which is best left to experts) and maintain their own miniature grinders to get the powder right before brewing of their favourite cuppa. If you are a ‘control freak’ and want to roast at home, be careful; read the next post for more.


Roasted chicory: Chicory is a widely and, sometimes uncontrolledly growing shrub, whose off-white abundant roots are an extremely interesting agri-product. The roots are washed thoroughly after harvesting, cut into stubs and sun-dried. They are then roasted to brown ‘roasted chicory root stubs’ but with a lot less care than coffee beans, given their lowly status. These are powdered to get roasted chicory root powder, simply called ‘chicory powder’ in trade. It closely resembles roasted coffee powder in every way and, not surprisingly, finds its abundant way into commercial coffee products including the ‘instant’ ones. Unless your coffee expressly makes ‘100% pure coffee’ claim, it contains chicory. Chicory offers some serious benefits, though not in the small amounts that come with coffee.


Roasted cocoa beans: Everything about coffee beans applies to it with just one major difference: cocoa beans contain the valuable ‘cocoa butter’ which is a part of the bean exactly as groundnut oil is a part of its seed or kernel. It is called ‘butter’ just to denote its consistency at normal temperature ranges. Moulded bar chocolates owe their ‘snap’ and textural eating experience to cocoa butter. This is a world in itself; suffice it to say that oxygen-mediated controlled roasting is crucial here also.
 Caramel: One of world’s favorite both as a coloring and flavoring agent, it has many culinary versions. Being practically dry, sugar does not have the protection of evaporating water when heated and hence gets heated quickly. It soon melts and turns brownish because of a series of complex reactions with atmospheric oxygen. It is a skill to stop heating at the right stage and is called ‘caramelization’ of sugar. In the wet process, water is added to sugar before burning; water evaporates quickly but creates a time window for better control. When this process is stopped with just minor browning, the product is called ‘caramelized sugar’. When chefs expertly burn sugar crystals on a set cold dessert with a torch (‘flambe’), it forms a ‘brown glass candy’ on the top without heating the basic dessert much. Some nutritive properties have been attributed to caramel.
Caramel: One of world’s favorite both as a coloring and flavoring agent, it has many culinary versions. Being practically dry, sugar does not have the protection of evaporating water when heated and hence gets heated quickly. It soon melts and turns brownish because of a series of complex reactions with atmospheric oxygen. It is a skill to stop heating at the right stage and is called ‘caramelization’ of sugar. In the wet process, water is added to sugar before burning; water evaporates quickly but creates a time window for better control. When this process is stopped with just minor browning, the product is called ‘caramelized sugar’. When chefs expertly burn sugar crystals on a set cold dessert with a torch (‘flambe’), it forms a ‘brown glass candy’ on the top without heating the basic dessert much. Some nutritive properties have been attributed to caramel.
In food science, the term caramelization is sometimes used to describe the heat- and oxygen-mediated browning of all carbohydrates. Though serious alarm has not been raised about it, doubts persist about carcinogenicity of caramel and its products or dishes. Of particular concern is acrylamide – a known carcinogen, also formed when potatoes are fried as in wafers and French fries. Alarming? Moderation is the name of the game.
(A stunning co-incidence: Coffee, chicory, cocoa, caramel – all processed silmilarly from similar starting materials. Have comparable color and flavor profile. All undergo varying degrees of aerial oxidation. An adventurous food scientist should have a crack at ‘cheat (and cheap) coffee’ made from roasted chicory extract and caramel.
 Miscellaneous:
Miscellaneous:
- Roasting eggplant or brinjal or aubergine on open flame (or in an oven) happens with the evaporating protection of water. However, this protection is not total which explains the development of mysterious flavor of the product. No need for alarm given the protection of water but don’t overdo it.
- Whole spices contain delicate volatiles apart from water and usual plant tissue. It is common to carefully dry them initially so that they become crisp enough to break easily. During such drying with air some development of flavours is possible. The process of grinding generates heat and hence most industrial grinding is done with indirect cooling of the grinder. Chefs routinely toast (i.e. heat gently to primarily dry, with little flavor development) whole spices before grinding. The resulting fragile and dry spice develops subtle flavours which can be attributed to reactions with oxygen.
- We use oxygen-mediated flavor (and color) development extensively in daily cooking. ‘Browning’ of onions, garlic, potatoes, bread slices, seeds like fenugreek, Bengal gram and Udad daal, etc. are examples. Note: (i) Incomplete combustion of food – a complex mix of largely organic molecules – produces mysterious flavours along with carcinogenic molecules. So the practice of ‘smoking’ food dishes at home (‘dhungar’ in most Indian languages) is dangerous. Note that carbon monoxide is the product of incomplete combustion of fuels. (ii) ‘Air friers’ are becoming popular for their limited use of cooking oils. Hot air brushing surface-oiled food articles can be dicey. This calls for a separate post but don’t use refined soybean oil for this. (iii)Usually, the things that can be browned easily have little free water; tomatoes cannot be browned easily.
Next post: Oxygen, food and life, Part II
The dark side of oxygen
(Visit ‘disclaimer’)
97 thoughts on “Oxygen, food and life : Part I (How oxygen mediates in life and food processing)”
Just wish to say your article is as astounding. The clarity in your post is just great and i can assume you are an expert on this subject. Fine with your permission allow me to grab your RSS feed to keep updated with forthcoming post. Thanks a million and please continue the enjoyable work.
Great content! Super high-quality! Keep it up!
Thank you for your post. I really enjoyed reading it, especially because it addressed my issue. It helped me a lot and I hope it will also help others.
Thanks for your help and for writing this post. It’s been great.
Great content! Super high-quality! Keep it up!
Thank you for your post. I really enjoyed reading it, especially because it addressed my issue. It helped me a lot and I hope it will also help others.
I like the valuable info you provide in your articles. I will bookmark your weblog and check again here regularly. I’m quite sure I will learn a lot of new stuff right here! Good luck for the next!
you have an important weblog here! would you like to make some invite posts on my blog?
Hi,
Thanks for the invitation, but we don’t do that. Please stay tuned for more posts on our blog.
Wow that was odd. I just wrote an incredibly long comment but after I clicked submit my comment didn’t show up. Grrrr… well I’m not writing all that over again. Anyhow, just wanted to say fantastic blog!
Yay google is my world beater assisted me to find this outstanding site! .
Thank you for sharing this article with me. It helped me a lot and I love it. http://www.ifashionstyles.com
The articles you write help me a lot and I like the topic http://www.kayswell.com
Thanks for your help and for writing this post. It’s been great. http://www.kayswell.com
Thanks for posting. I really enjoyed reading it, especially because it addressed my problem. http://www.kayswell.com It helped me a lot and I hope it will help others too.
May I request more information on the subject? http://www.kayswell.com All of your articles are extremely useful to me. Thank you!
Thank you for your post. I really enjoyed reading it, especially because it addressed my issue. http://www.kayswell.com It helped me a lot and I hope it will also help others.
How can I find out more about it? http://www.kayswell.com
Can you write more about it? Your articles are always helpful to me. Thank you! http://www.kayswell.com
Thank you for another fantastic post. Where else could anyone get that type of information in such a perfect way of writing? I’ve a presentation next week, and I’m on the look for such info.
Thanks for posting. I really enjoyed reading it, especially because it addressed my problem. http://www.hairstylesvip.com It helped me a lot and I hope it will help others too.
You helped me a lot with this post. http://www.kayswell.com I love the subject and I hope you continue to write excellent articles like this.
You helped me a lot with this post. http://www.kayswell.com I love the subject and I hope you continue to write excellent articles like this.
Thank you for your help and this post. It’s been great. http://www.kayswell.com
Please tell me more about your excellent articles http://www.ifashionstyles.com
Can you write more about it? Your articles are always helpful to me. Thank you! http://www.kayswell.com
Thank you for your post. I really enjoyed reading it, especially because it addressed my issue. http://www.kayswell.com It helped me a lot and I hope it will also help others.
Thank you for writing this post. I like the subject too. http://www.kayswell.com
Thanks for posting. I really enjoyed reading it, especially because it addressed my problem. http://www.hairstylesvip.com It helped me a lot and I hope it will help others too.
May I request more information on the subject? http://www.ifashionstyles.com All of your articles are extremely useful to me. Thank you!
Sustain the excellent work and producing in the group! http://www.hairstylesvip.com
Thank you for your articles. I find them very helpful. Could you help me with something? http://www.kayswell.com
Please provide me with more details on the topic http://www.kayswell.com
Thank you for being of assistance to me. I really loved this article. http://www.kayswell.com
Thank you for writing this post! http://www.hairstylesvip.com
Thank you for your post. I really enjoyed reading it, especially because it addressed my issue. http://www.kayswell.com It helped me a lot and I hope it will also help others.
I really like what you guys tend to be up too. This kind of clever work and reporting! Keep up the amazing works guys I’ve added you guys to my blogroll.
Thank you for writing this post! http://www.kayswell.com
That is a very good tip especially to those new to the blogosphere. Short but very accurate information… Many thanks for sharing this one. A must read article! http://www.kayswell.com
Hi there, just became alert to your blog through Google,and found that it is truly informative. I am gonna watch outfor brussels. I’ll be grateful if you continue this in future.Lots of people will be benefited from your writing.Cheers! http://www.kayswell.com
It’s not my first time to visit this web site, i am browsing this website dailly and get good facts from here all the time. http://www.kayswell.com
Aw, this was a very nice post. Taking the time and actual effort to create a great article… but what can I say… I hesitate a whole lot and never seem to get nearly anything done. http://www.kayswell.com
Hello there, You’ve done a great job. I will certainly digg it and personally recommend to my friends. I’m sure they will be benefited from this site. http://www.kayswell.com
Superb post however , I was wanting to know if you could write a litte more on this subject? I’d be very grateful if you could elaborate a little bit further. http://www.kayswell.com
I feel this is one of the such a lot important information for me. And i’m satisfied reading your article. But should observation on some normal issues, The site style is perfect, the articles is actually nice : http://www.kayswell.com
Ahaa, its good dialogue regarding this article at this place at this website, I have read all that, so at this time me also commenting here. http://www.kayswell.com
These are actually great ideas in about blogging. You have touched some pleasant things here. Any way keep up wrinting.
Ahaa, its fastidious discussion on the topic of this article at this place at this website, I have read all that, so now me also commenting at this place. http://www.kayswell.com
Have you ever thought about adding a little bit more than just your articles? I mean, what you say is valuable and everything. Nevertheless think about if you added some great pictures or video clips to give your posts more, http://www.kayswell.com“pop”! Your content is excellent but with pics and videos, this site could certainly be one of the best in its niche. Terrific blog!
Great blog you’ve got here.. It’s difficult to find excellent writing like yours these days. I seriously appreciate individuals like you! Take care!! http://www.kayswell.com
Spot on with this write-up, I really believe this site needs much more attention. I’ll probably be returning to read through more, thanks for the info! http://www.kayswell.com
Fantastic blog! Do you have any tips for aspiring writers? I’m planning to start my own website soon but I’m a little lost on everything. Would you suggest starting with a free platform like WordPress or go for a paid option? http://www.kayswell.com There are so many options out there that I’m totally confused .. Any ideas?
I’m not that much of a internet reader to be honest but your blogs really nice, keep it up! I’ll go ahead and bookmark your site to come back down the road. Many thanks
Now I am ready to do my breakfast, once having my breakfast coming over again to read more news http://www.kayswell.com
It’s not my first time to visit this web site, i am browsing this website dailly and get good facts from here all the time. http://www.hairstylesvip.com
I’ve been surfing online more than 3 hours today, yet I never found any interesting article like yours. It’s pretty worth enough for me. Personally, if all webmasters and bloggers made good content as you did, the web will be much more useful than ever before. http://www.kayswell.com
I am genuinely thankful to the owner of this web page who has shared this fantastic post at here. http://www.hairstylesvip.com
Right now it sounds like WordPress is the preferred blogging platform out there right now. (from what I’ve read) Is that what you are using on your blog? http://www.ifashionstyles.com
Yes.
Fantastic blog! Do you have any tips for aspiring writers? I’m planning to start my own website soon but I’m a little lost on everything. Would you suggest starting with a free platform like WordPress or go for a paid option? http://www.kayswell.com There are so many options out there that I’m totally confused .. Any ideas?
Hi,
We are using WordPress for our website.
Stay tuned for future posts.
Nice answers in return of this matter with firm arguments and telling everything concerning that. http://www.kayswell.com
Have you ever considered about including a little bit more than just your articles? I mean, what you say is fundamental and all. However imagine if you added some great images or video clips to give your posts more, “pop”! Your content is excellent but with images and clips, http://www.kayswell.com this website could undeniably be one of the best in its field.
Aw, this was a very nice post. Taking the time and actual effort to create a great article… but what can I say… I hesitate a whole lot and never seem to get nearly anything done.
We stumbled over here coming from a different web page and thought I might check things out. I like what I see so now i’m following you.Look forward to finding out about your web page yet again. http://www.ifashionstyles.com
You actually make it seem so easy with your presentation but I find this matter to be actually something that I think I would never understand. It seems too complex and very broad for me. I’m looking forward for your next post, I will try to get the hang of it! http://www.kayswell.com
Have you ever thought about adding a little bit more than just your articles? I mean, what you say is valuable and everything. Nevertheless think about if you added some great pictures or video clips to give your posts more, http://www.kayswell.com“pop”! Your content is excellent but with pics and videos, this site could certainly be one of the best in its niche. Terrific blog!
We’re a group of volunteers and starting a new scheme in our community. Your website offered us with helpful information to work on. You’ve done a formidable task and our whole community will be thankful to you. http://www.kayswell.com
This is my first time visit at here and i am actually happy to read everthing at alone place. http://www.kayswell.com
Hi, Neat post. There is a problem with your site in web explorer, might test this? IE still is the market chief and a large component of other people will leave out your wonderful writing because of this problem. http://www.hairstylesvip.com
What’s Going down i’m new to this, I stumbled upon this I have discovered It absolutely useful and it has aided me out loads. I am hoping to give a contribution & assist other users like its helped me. http://www.ifashionstyles.com Great job.
I really like what you guys tend to be up too. This kind of clever work and reporting! Keep up the amazing works guys I’ve added you guys to my blogroll. http://www.ifashionstyles.com
I am not sure where you’re getting your information, but great topic. I needs to spend some time learning more or understanding more. Thanks for excellent info I was looking for this information for my mission.
Oh my goodness! Incredible article dude! Many thanks, However I am experiencing problems with your RSS. I don’t understand the reason why I cannot join it. Is there anybody having the same RSS issues? Anyone that knows the solution will you kindly respond? Thanx!!
What i do not understood is actually how you’re no longer really much more well-preferred than you may be now. You’re very intelligent. You know thus significantly in terms of this subject, made me personally believe it from a lot of numerous angles. Its like men and women aren’t interested unless it’s something to do with Girl gaga! Your individual stuffs great. At all times deal with it up!
I used to be able to find good advice from your blog articles.
These are actually great ideas in about blogging. You have touched some pleasant things here. Any way keep up wrinting.
Wonderful goods from you, man. I’ve understand your stuff previous to and you’re just too excellent.I really like what you have acquired here, certainly likewhat you are stating and the way in which you say it.You make it entertaining and you still care for to keep it wise.I cant wait to read much more from you. This is actually a wonderful site.
I really like what you guys tend to be up too. This kind of clever work and reporting! Keep up the amazing works guys I’ve added you guys to my blogroll.
I constantly emailed this blog post page to all my contacts, for the reason that if like to read it after that my friends will too.
Do you mind if I quote a few of your articles as long as I provide credit and sources back to your website? My website is in the exact same area of interest as yours and my visitors would definitely benefit from some of the information you provide here. http://www.ifashionstyles.com
Hi,
Our articles are proprietary. Please don’t forget to quote the source – our website https://www.letfoorliftlife.com – if you do use our articles anywhere else on the internet. Else, it will be a violation of our intellectual property. Hope you continue to enjoy and learn from our articles.
Thanks and regards.
You actually make it seem so easy with your presentation but I find this matter to be actually something that I think I would never understand. It seems too complex and very broad for me. I’m looking forward for your next post, I will try to get the hang of it! http://www.hairstylesvip.com
I think the admin of this website is in fact working hard in support of his website, as here every stuff is quality based stuff.
I like the valuable info you provide to your articles. I will bookmark your weblog and check once more here regularly. I am reasonably sure I’ll be told lots of new stuff proper right here! Best of luck for the following!
I like the valuable info you provide to your articles. I will bookmark your weblog and check once more here regularly. I am reasonably sure I’ll be told lots of new stuff proper right here! Best of luck for the following! http://www.kayswell.com
Your mode of explaining everything in this piece of writing is genuinely pleasant, every one be capable of simply be aware of it, Thanks a lot. http://www.kayswell.com
I’m not that much of a internet reader to be honest but your blogs really nice, keep it up! I’ll go ahead and bookmark your website to come back in the future. http://www.kayswell.com
I am really impressed with your writing skills as well as with the layout on your blog. Is this a paid theme or did you modify it yourself? Anyway keep up the nice quality writing, it is rare to see a nice blog like this one nowadays. http://www.hairstylesvip.com
Hurrah! In the end I got a weblog from where I know how to truly take helpful information regarding my study and knowledge.
Hello, I enjoy reading through your article. I wanted to write a little comment to support you. http://www.hairstylesvip.com
I just like the valuable information you provide on your articles.I’ll bookmark your blog and test again here frequently.I’m quite certain I’ll be informed plenty of new stuff proper right here! Good luck for the next!
Do you mind if I quote a few of your articles as long as I provide credit and sources back to your website? My blog site is in the exact same area of interest as yours and my visitors would really benefit from a lot of the information you provide here.Please let me know if this ok with you. Regards!
Hello, I enjoy reading all of your post. I wanted to write a little comment to support you. http://www.kayswell.com
Somebody essentially assist to make critically posts I would state. That is the first time I frequented your website page and to this point? I amazed with the research you made to make this actual put up amazing. Magnificent task!